So, we’ve identified the chemical structures of starch, cellulose, and hemicellulose. Now we’re going to look at what lignin is, chemically.
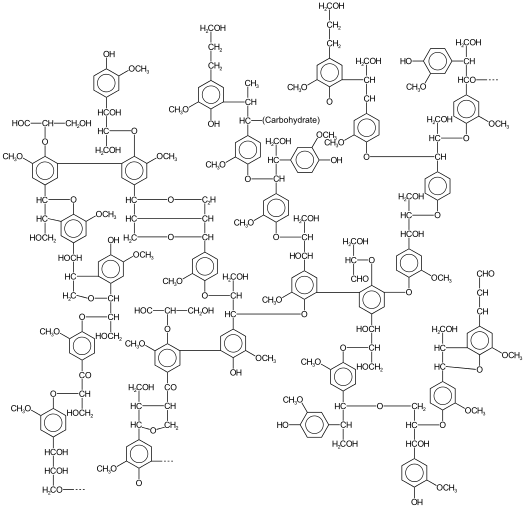
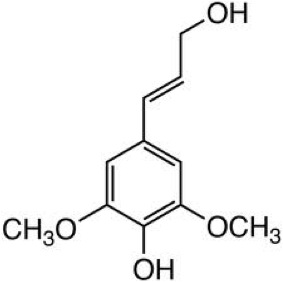
Vascular land plants make lignin to solve problems due to terrestrial lifestyles. Lignin helps to keep water from permeating the cell wall, which helps water conduction in the plant. Lignin adds support – it may help to “weld” cells together and provides stiffness for resistance against forces that cause bending, such as wind. Lignin also acts to prevent pathogens and is recalcitrant to degradation; it protects against fungal and bacterial pathogens (there is a discussion in Lesson 5 about recalcitrance). Lignin is comprised of crosslinked, branched aromatic monomers: p-coumaryl alcohol, coniferyl alcohol, and sinapyl alcohol; their structures are shown in the figures below and show how these building blocks fit into the lignin structure. p-Coumaryl alcohol is a minor component of grass and forage-type lignins. Coniferyl alcohol is the predominant lignin monomer found in softwoods (hence the name). Both coniferyl and sinapyl alcohols are the building blocks of hardwood lignin. The table below shows the differing amounts of lignin building blocks in the three types of lignocellulosic biomass sources.
| Lignin Sources | Grasses | Softwood | Hardwood |
|---|---|---|---|
| p-coumaryl alcohol | 10-25% | 0.5-3.5% | Trace |
| coniferyl alcohol | 25-50% | 90-95% | 25-50% |
| sinapyl alcohol | 25-50% | 0-1% | 50-75% |


Several different materials can be made from lignin, but most are not on a commercial scale. The table below shows the class of compounds that can be made from lignin and the types of products that come from that class of compounds. If an economic method can be developed for lignin depolymerization and chemical production, it would benefit the biorefining of lignocellulosic biomass.
| Class of Compound | Product Examples |
|---|---|
| Simple aromatics | Biphenyls, Benzene, Xylenes |
| Hydroxylated aromatics | Phenol, Catechol, Propylphenol, etc. |
| Aromatic Aldehydes | Vanillin, Syringaldehyde |
| Aromatic Acids and Diacids | Vanillic Acid |
| Aliphatic Acids | Polyesters |
| Alkanes | Cyclohexane |
There are also high molecular weight compounds. These include carbon fibers, thermoplastic polymers, fillers for polymers, polyelectrolytes, and resins, which can be made into wood adhesives and wood preservatives.