Lesson 8: Biodiesel Production
Lesson 8: Biodiesel Production mjg8Overview
We’ve focused on the processes used to make ethanol from fermentation and to create other products using thermochemical methods. Now we will look at making biodiesel from fats using transesterification as well as other methods being investigated. Converting vegetable oils into biodiesel is fairly straightforward and easy to do, but it is still not very economical. We will begin with a brief chemistry tutorial on the chemistry of vegetable oils and animal fats before going into transesterification. We will then discuss other methods of biodiesel production.
Lesson Objectives
By the end of this lesson, you should be able to:
- explain the chemistry of vegetable oil biodiesel, and several ways to make it;
- explain how to utilize biodiesel in a diesel engine;
- evaluate the best uses of biodiesel.
Lesson 8 Road Map
This lesson will take us one week to complete. Please refer to the Course Syllabus for specific time frames and assignment due dates.
Questions?
If there is anything in the lesson materials that you would like to comment on or don't quite understand, please post your thoughts and/or questions to our Throughout the Course Questions and Comments discussion forum. The discussion forum will be checked regularly. While you are there, feel free to post responses to your classmates if you are able to help. Regular office hours will be held to provide help for EGEE 439 students.
8.1 Terminology for Vegetable Oils and Animal Fats
8.1 Terminology for Vegetable Oils and Animal Fats mjg8Fat is a generic term for lipids, a class of compounds in biochemistry. You would know them as greasy, solid materials found in animal tissues and in some plants – oils that are solids at room temperature.
Vegetable oil is the fat extracted from plant sources. We may be able to extract oil from other parts of a plant, but seeds are the main source of vegetable oil. Typically, vegetable oils are used in cooking and for industrial uses. Compared to water, oils and fats have a much higher boiling point. However, there are some plant oils that are not good for human consumption, as the oils from these types of seeds would require additional processing to remove unpleasant flavors or even toxic chemicals. These include rapeseed and cottonseed oil.
Animal fats come from different animals. Tallow is beef fat and lard is pork fat. There is also chicken fat, blubber (from whales), cod liver oil, and ghee (which is a butterfat). Animal fats tend to have more free fatty acids than vegetable oils do.
Chemically, fats and oils are also called “triglycerides.” They are esters of glycerol, with a varying blend of fatty acids. The figure below shows a generic diagram of the structure without using chemical formulas.

So what is glycerol? It is also known as glycerin/glycerine. Other names for glycerol include 1,2,3-propane-triol, 1,2,3-tri-hydroxy-propane, glyceritol, and glycyl alcohol. It is a colorless, odorless, hygroscopic (i.e., will attract water), and sweet-tasting viscous liquid. The following figure shows the chemical structure in two different forms.

So now we need to define what the fatty acids are. Essentially, fatty acids are long-chain hydrocarbons with a carboxylic acid. The following figure shows the generic chemical structure of a fatty acid with the carboxylic acid on it.

This image provides a visual and textual explanation of the carboxylic acid functional group, commonly found in organic molecules such as fatty acids. It features two equivalent representations of the same chemical structure:
- R–C=O with an –OH group attached to the carbon, which is the expanded structural form.
- RCOOH, the condensed molecular formula.
In both cases, "R" denotes a long hydrocarbon chain, which can vary in structure. The image notes that this chain may be saturated, meaning it contains only single bonds between carbon atoms, or unsaturated, meaning it includes one or more double bonds.
The COOH group is identified as the acidic functional group—a defining feature of carboxylic acids. The image emphasizes that this group can be written in either the expanded or condensed form, both of which are chemically equivalent.
The figure below shows different fatty acid chemical structures. The chemical structures are shown as line chemical structures, where each point on the links is a carbon atom and the correct number of hydrogen atoms is dependent on whether there is a single or double bond. Fatty acids can be saturated (with hydrogen bonds) or unsaturated (with some double bonds between carbon atoms). Because of the metabolism of oilseed crops, naturally formed fatty acids contain even numbers of carbon atoms. In organic chemistry, carbon atoms have four pairs of electrons available to share with another carbon, hydrogen, or oxygen atom. Free fatty acids are not bound to glycerol or other molecules. They can be formed from the breakdown or hydrolysis of a triglyceride.




The fatty acids shown have slightly different properties. Palmitic acid is found in palm oil. The figure below shows the relationship of each fatty acid to its size and saturation. Palmitic and steric acids are saturated fatty acids, while oleic and linoleic acids are unsaturated with different amounts of double bonds. The figure below shows differing amounts of carbon atoms compared to the number of double bonds in the compound.

The figure below shows the part of the triglyceride that is a fatty acid and the part that is glycerol, including chemical structures this time. The chemical structure shown here is a saturated triglyceride.
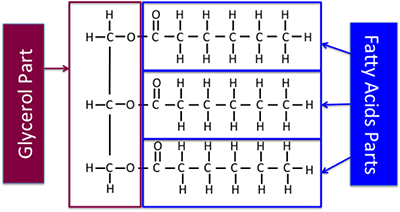
So, we’ve discussed what fats and oils are. Now, what is biodiesel? What is at least one definition? It is a diesel fuel that was generated from biomass. However, there are different types of biodiesel. The most commonly known type of biodiesel is a fuel comprised of mono-alkyl esters (typically methyl or ethyl esters) of long-chain fatty acids derived from vegetable oils or animal fats – this is according to ASTM D6551. An ASTM is a document that contains the standards for particular types of chemicals, particularly industrial materials. This is a wordy definition that doesn’t really show us what it is chemically.
So when we talk about an alkyl group, it is a univalent radical containing only carbon and hydrogen atoms in a hydrocarbon chain, with a general atomic formula of CnH2n+1. Examples include:
| Alkyl Name | Chemical Derived From | Chemical Formula |
|---|---|---|
| Methyl | Derived from methane | CH3- |
| Ethyl | Derived from ethane | CH3CH2- |
Another term we need to know about is an ester. Esters are organic compounds where an alkyl group replaces a hydrogen atom in a carboxylic acid. For example, if the acid is acetic acid and the alkyl group is the methyl group, the resulting ester is called methyl acetate. The reaction of acetic acid with methanol will form methyl acetate and water; the reaction is shown below. An ester formed in this method is a condensation reaction; it is also known as esterification. These esters are also called carboxylate esters.
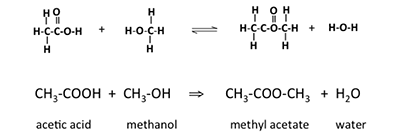
This image illustrates a chemical esterification reaction, both in terms of structural formulas and molecular formulas, showing the formation of an ester from a carboxylic acid and an alcohol.
At the top, the structural formulas depict the reaction between acetic acid (CH₃COOH) and methanol (CH₃OH). The acetic acid molecule features a carboxyl group (–COOH), while methanol contains a hydroxyl group (–OH). These two reactants undergo a condensation reaction, where a molecule of water (H₂O) is eliminated, and a new bond forms between the acid and alcohol.
The product of this reaction is methyl acetate (CH₃COOCH₃), an ester, along with water. The reaction is shown as reversible, indicated by the double arrows (⇌), which is characteristic of esterification reactions under equilibrium conditions.
Below the structural representation, the molecular formulas summarize the same reaction:
Each compound is labeled:
- Acetic acid
- Methanol
- Methyl acetate
- Water
This is the basic reaction that helps to form biodiesel. The following figure shows the different parts of the chemical structure of the biodiesel, the methyl ester fatty acid, or fatty acid methyl ester (FAME).

So, at this point, let’s make sure we know what we have been discussing. Biodiesel is a methyl (or ethyl) ester of a fatty acid. It is made from vegetable oil, but it is not vegetable oil. If we have 100% biodiesel, it is known as B100 – it is a vegetable oil that has been transesterified to make biodiesel. It must meet ASTM biodiesel standards to qualify for warranties sold as biodiesel and qualify for any tax credits. Most often, it is blended with petroleum-based diesel. If it is B2, it has 2% biodiesel and 98% petroleum-based diesel. Other blends include B5 (5% biodiesel), B20 (20% biodiesel), and B100 (100% biodiesel). We’ll discuss why blends are used in the following section. And to be clear: sometimes vegetable oil is used in diesel engines, but it can cause performance problems and deteriorate engines over time. Sometimes, vegetable oil and alcohol are mixed together in emulsions, but that it is still not biodiesel, as it has different properties from biodiesel.
So, if straight vegetable oil (SVO) will run in a diesel engine, why not use it? Vegetable oil is significantly more viscous (gooey is a non-technical term) and has poorer combustion properties. It can cause carbon deposits, poor lubrication within the engine, and engine wear, and it has cold-starting problems. Vegetable oils have natural gums that can cause plugging in filters and fuel injectors. For a diesel engine, the injection timing is thrown off and can cause engine knocking. There are ways to mitigate these issues, which include: 1) blending with petroleum-based diesel (usually < 20%), 2) preheating the oil, 3) making microemulsions with alcohols, 4) “cracking” the vegetable oil, and 5) using the method of converting SVO into biodiesel using transesterification. Other methods are used as well, but for now, we’ll focus on biodiesel from transesterification. The table below shows three properties of No. 2 diesel, biodiesel, and vegetable oil. As you can see, the main change is in the viscosity. No. 2 Diesel and biodiesel have similar viscosities, but vegetable oils have much higher viscosity and can cause major problems in cold weather. This is the main reason for converting the SVO into biodiesel.
| Fuel | Energy Content (Btu/gal) | Cetane Number | Viscosity (centistokes) |
|---|---|---|---|
| No. 2 Diesel | 140,000 | 48 | 3 |
| Biodiesel | 130,000 | 55 | 5.7 |
| Vegetable oil | 130,000 | 50 | 45 |
8.2 The Reaction of Biodiesel: Transesterification
8.2 The Reaction of Biodiesel: Transesterification mjg8So, how do we make biodiesel?
The method being described here is for making FAMEs biodiesel. The reaction is called transesterification, and the process takes place in four steps. The first step is to mix the alcohol for reaction with the catalyst, typically a strong base such as NaOH or KOH. The alcohol/catalyst is then reacted with the fatty acid so that the transesterification reaction takes place. The first figure below shows the preparation of the catalyst with the alcohol, and the second figure shows the transesterification reaction.

Formation of methoxide

The catalyst is prepared by mixing methanol and a strong base such as sodium hydroxide or potassium hydroxide. During the preparation, the NaOH breaks into ions of Na+ and OH-. The OH- abstracts the hydrogen from methanol to form water and leaves the CH3O- available for reaction. Methanol should be as dry as possible. When the OH- ion reacts with H+ ion, it reacts to form water. Water will increase the possibility of a side reaction with free fatty acids (fatty acids that are not triglycerides) to form soap, an unwanted reaction. Enzymatic processes can also be used (called lipases); alcohol is still needed and only replaces the catalyst. Lipases are slower than chemical catalysts, are high in cost, and produce low yields.
Once the catalyst is prepared, the triglyceride will react with 3 mols of methanol, so excess methanol has to be used in the reaction to ensure a complete reaction. The three attached carbons with hydrogen react with OH- ions and form glycerin, while the CH3 group reacts with the free fatty acid to form the fatty acid methyl ester.
The figure below is a graphic of the necessary amounts of chemicals needed to make the reaction happen and the overall yield of biodiesel and glycerin. The amount of methanol added is almost double the required amount so the reaction goes to completion. With 100 lbs of fat and 16-20 lbs of alcohol (and 1 lb of catalyst), the reaction will produce 100 lbs of biodiesel and 10 lbs of glycerin. The reaction typically takes place at between 40-65°C. As the reaction temperature goes higher, the rate of reaction will increase, typically 1-2 hours at 60 °C versus 2-4 hours at 40°C. If the reaction is higher than 65°C, a pressure vessel is required because methanol will boil at 65°C. It also helps to increase the methanol-to-oil ratio. Doubling the ratio of 3 mols of alcohol to 6 mols will push the reaction to completion faster and more completely.

The following video shows a time-lapsed reaction of transesterification of vegetable oil into biodiesel. It also incorporates the steps after the reaction to separate out the biodiesel (9:44).
Making biodiesel
MARK HALL: Hello. I'm Mark Hall of the Auburn University Extension Renewable Energy Specialist. We're doing several of these things on energy options that you can do, several pieces that, each piece of the puzzle, that you can contribute to our energy independence by making ethanol, making biodiesel, being more energy efficient in how you operate your home.
Today, we're going to talk about making biodiesel. And we have Lance Hall. Lance has been making biodiesel to run in his car. He bought a used Volkswagen off eBay and started making biodiesel. And he's liked it so much that he's bought a new diesel car. And he's been real successful doing this for a couple of years.
Before we bring Lance in, I'd like to thank my friend and coworker Walter Harris, the county agent coordinator in Madison County, for filming us today. Lance, come in and show us what you've been doing. And congratulations. You've been successful doing this.
I was talking to my daddy about my new job several years ago. And he said, well, Lance has been doing that for a long time. I said, what? I didn't know that. So Lance, show people how to make biodiesel.
LANCE HALL: OK. A lot of people know about the biodiesel. They've read the stories. They've done some research. But, yet, they still don't have enough confidence in their ability to actually make a batch. So I'm going to show you today on how to make a batch of biodiesel, just small scale, but it's easy.
OK. The first thing that we're going to do is start off with vegetable oil. Now, this will be 800 milliliters. And don't be confused between the milliliters and your normal units of measure. It's a simple conversion that anybody can do with a handheld calculator.
So we've got 800 milliliters here. Well, first thing we want to do is heat it. Now, don't be concerned about this fancy piece of equipment, either. The main element of this is to heat it any way that you can safely.
And these things here are magnetic stirrers. Again, don't be concerned with this. Just stir it while you're heating it to even things out. And we're going to heat this up to about 130 degrees Fahrenheit.
MARK HALL: Lance, tell them about where you get this equipment.
LANCE HALL: All of this equipment that I've got in my shop, all my lab stuff, eBay is a wonderful place to find a used lab supplies, lab glass. These are magnetic stirrer plates. These are really handy to have if you have the means to buy them. You don't have to have them, of course. But I like to use them.
And this is also an electronic scale that comes in handy when you start weighing out your catalyst, doing anything that you want to measure a precise weight. That's worth the money there. And that's going to take a little while, so--
MARK HALL: Lance, is there any other sites, internet sites that you would recommend for people that are interested in making biodiesel?
LANCE HALL: There are several sites out there. One of the most informative on what biodiesel is, where it's being used, is biodiesel.org. That's the National Biodiesel Board website, lots of good information there. It won't really tell you as much how to make it, but hopefully, this will be one of the more informative sites that you'll actually be able to see somebody make one, make a batch.
OK. As our oil is heating up, we have to mix up our methanol potassium hydroxide mixture. So safety is paramount with the use of methanol or the strong caustic lye potassium hydroxide. Methanol can cause blindness or death, and it can be absorbed through the skin. And the potassium hydroxide will burn your skin if it gets on you.
So here's what we're going to do. We're going to take our methanol and we're going to pour this into a container. Face shields are good, too.
We're going to use 175 milliliters of the methanol. That's roughly 20% of the 800 milliliters of oil. You usually want to use about a 20% methanol volume compared to the veggie oil volume.
OK. Our next ingredient is our potassium hydroxide. That's our lye. Now, we have to do a quick calculation on how much of this we need to mix with our methanol in order for the reaction to take place.
I've got a nice spreadsheet that I like to use. It's the Biodiesel-o-matic. You can usually find it online from different biodiesel websites. I'm going to pull that up.
OK. We want to use 7 grams of potassium hydroxide per each liter of veggie oil. So you take 7 divided by 0.8. And that gives you 6.4 grams.
Double bag this stuff, or it will absorb moisture. And that will kill your process.
So we're going to use our scale. We're going to zero the container. And then we're going to put 6.4 grams into it. Make sure you have your gloves on.
OK. That's our 6.4 grams. Close this immediately. Keep it double-bagged. OK. Now, you're going to take your 6.4 grams of potassium hydroxide and put that into your 175 milliliters of methanol.
Again, you want to stir this. It's not necessary to heat it, though. Just stir. And stir this until at least the potassium hydroxide is completely dissolved into the methanol. You don't want to see any chunks of white potassium hydroxide flakes.
All right. Our potassium hydroxide is fully mixed into our methanol. We want to remove the stir bar. And then we're just going to slowly pour this into our oil as it's being stirred.
Again, you don't have to have fancy equipment. Just pour it in as you're stirring it manually. But the key is to do it slowly.
The figure below shows a schematic of the process for making biodiesel. Glycerol is formed and has to be separated from the biodiesel. Both glycerol and biodiesel need to have alcohol removed and recycled in the process. Water is added to both the biodiesel and glycerol to remove unwanted side products, particularly glycerol, that may remain in the biodiesel. The wash water is separated out similar to solvent extraction (it contains some glycerol), and the trace water is evaporated out of the biodiesel. Acid is added to the glycerol in order to provide neutralized glycerol.

Schematic of the biodiesel process using transesterification.
Schematic of the biodiesel process using transesterification:
Oil, alcohol, and a catalyst undergo transesterification. From there they are mixed methyl esters from which crude glycerol is removed. The crude glycerol goes into a separator under heat and a vacuum in which alcohol is removed. It then goes through a water wash and is neutralized with acid to produce neutralized glycerol. The other remaining mixed methyl esters from transesterification go into a different separator which removes any alcohol. They then undergo an extraction using water and move into a second separator under heat and a vacuum that removes any water. This yields biodiesel.
As briefly discussed, the initial reactants used in the process should be as dry as possible. Water can react with the triglyceride to make free fatty acids and a diglyceride. It can also dissociate the sodium or potassium from the hydroxide, and the ions Na+ and K+ can react with the free fatty acid to form soap. The figure below shows how water can help to form a free fatty acid, and that free fatty acid can react with the Na+ ion to form soap. The sodium that was being used for a catalyst is now bound with the fatty acid and unusable. It also complicates separation and recovery. All oils may naturally contain free fatty acids. The refined vegetable oil contains less than 1%, while crude vegetable oil has 3%, waste oil has 5%, and animal fat has 20%. Animal fats are a less desirable feedstock.

This image illustrates two fundamental chemical processes involving triglycerides: hydrolysis and saponification, both of which are essential in lipid chemistry and soap production.
Part A: Hydrolysis of Triglycerides
In this reaction, a triglyceride—a molecule composed of a glycerol backbone esterified with three fatty acids—is partially hydrolyzed by water. The structural formula of the triglyceride is:
When water (H₂O) is added, one ester bond is cleaved, producing a free fatty acid and a diglyceride. The reaction is reversible and can be represented as:
Part B: Saponification (Soap Formation)
This part shows the saponification reaction, where a free fatty acid reacts with sodium hydroxide (NaOH). First, NaOH dissociates in water:
Then, the hydroxide ion reacts with the fatty acid:
Finally, the sodium ion combines with the carboxylate to form soap:
This reaction is the basis of traditional soap-making, converting fats into glycerol and soap through alkaline hydrolysis.
8.3 Various Processes Used to Make Biodiesel
8.3 Various Processes Used to Make Biodiesel mjg8Some of the processes used in making biodiesel are different from what we’ve discussed. The first of these processes we’ll discuss is solvent extraction.
In the process of making biodiesel through transesterification, we noted that biodiesel and glycerol are the products, with some water formation and unwanted potential soap formation. So, the products are liquid, but they are also immiscible (do not dissolve in each other) and have differences in specific gravity. The specific gravity of the products is shown in the table below.
| Material | Specific gravity (g/cm3) |
|---|---|
| Glycerol (pure) | 1.26 |
| Glycerol (crude) | 1.05 |
| Biodiesel | 0.88 |
| Methanol | 0.79 |
In batch processing, gravity separation is used, and the products remain in the reactor; the reactor then becomes a settler or decanter. Once the reaction is finished, the product mixture then sits without agitation. After 4-8 hours, the glycerol layer settles at the bottom (because it has higher gravity) and the biodiesel settles at the top. However, if a continuous flow facility is utilized, the products separate too slowly in a settler, so a centrifuge is used. A centrifuge will spin the liquids at a very high speed, which helps to promote density separation. The figure below shows a few different types of industrial centrifuges that can be used for biodiesel separation.
One of the issues that can happen during separation is the forming of a layer containing water and soap, in between the glycerol and biodiesel. That will hinder the separation. Another issue is that glycerol contains 90% of the catalyst and 70% of the excess methanol. In other words, the glycerol fraction is kind of the “trashcan” layer of the process. The biodiesel layer also contains some contaminants, including soap, residual methanol, free glycerol, and residual catalyst. The catalyst in biodiesel is extremely problematic if introduced into fuel systems. One way to improve the separation is through water washing with hot water, as the contaminants are soluble in water, but the biodiesel is not. Water washing will remove contaminants such as soap, residual methanol, free glycerol, and catalysts. The water should be softened (had ions removed) and be hot (both the biodiesel and water should be at 60°C). Thorough mixing with the wash water is needed so that all the contaminants can be removed, but the mixing intensity should also be controlled so that emulsions do not form between the biodiesel and water. Sometimes acid is added in the wash process to separate out the soaps. However, the last portion of washing needs to be acid-free, so a step may need to be added to neutralize the glycerol.
There is more than one way to implement the washing process. For batch processes, two of the methods are: a) top spray and b) air bubbling (see figure below). For the top spray, a fine mist of water is sprayed top-down in a fine mist. The water droplets contact the biodiesel as the water flows down, separating out the impurities. Air bubbling is a method that uses air as a mobile phase. Air bubbles through a layer of water and carries water with it on the way up. As the air bubbles burst on the way up, water droplets are released and drop down on the biodiesel at the bottom, contacting the biodiesel and washing out impurities. It can be a relatively slow process; a combination of the two is also possible.

For continuous-flow processes, different equipment is used, which typically incorporates some sort of counter-current flow process. The lighter biodiesel is introduced at the bottom and the heavier water is introduced at the top, and as they flow the fluids contact each other so that the biodiesel at the top has impurities removed and the water flowing down out the bottom contains the contaminants. The figure below shows two types of counter-current units: a) counter-flow washing system and b) rotating disc extractor. Both units contain materials to increase the interaction between water and biodiesel. For the counter-flow system, packing increases the interaction, while for the rotating disk extractor, disks rotate around as the fluid flows through. These types of equipment are typically used on an industrial scale and need precise mechanical design and process control; these units cost much more than the other type of system.

This image presents two schematic diagrams labeled (a) and (b), each illustrating a different method for washing biodiesel using softened water to remove impurities, such as residual catalysts, soaps, and glycerol.
(a) Counter Flow Washing Column
This diagram shows a vertical column where two fluids—softened water and biodiesel—flow in opposite directions:
- Softened water enters from the top left and flows downward.
- Biodiesel enters from the bottom left and flows upward.
- As the two fluids pass each other in opposite directions, impurities are transferred from the biodiesel into the water.
- The cleaned biodiesel exits from the top right, while the gray water (now containing the impurities) exits from the bottom right.
This counter-current setup enhances mass transfer efficiency, making it a common method in biodiesel purification.
(b) Rotating Disk Extractor
This diagram depicts a more mechanically intensive system:
- Softened water is introduced from the top center.
- Biodiesel enters from the middle left.
- Inside the extractor, rotating disks create turbulence and increase the contact surface area between the two liquids, improving the extraction of impurities.
- The purified biodiesel exits from the top right, while the gray water exits from the bottom right.
The most problematic step in biodiesel production, however, is water washing. It requires heated, softened water, some method of wastewater treatment, and water/methanol separation. Methanol recovery from water is somewhat costly using methanol-water rectification. Water can also be removed by vacuum drying. One of the alternative methods for removing water is the use of absorbent materials such as magnesium silicate. One company that provides a process for doing this is Magnesol, which is produced by the Dallas Group. Once the magnesium silicate removes the water, it can be regenerated by heating it up and evaporating the water. Methanol must also be removed from the biodiesel; one method for doing this is flash vaporization of methanol.
So, which type of process should be used? Should it be a batch or continuous flow system? Smaller plants are typically batch (< 1 million gallons/yr). They do not require continuous operation 24 hours per day 7 days a week. The batch system provides better flexibility and the process can be tuned based on particular feedstocks. However, in a commercial, industrial setting, most likely a continuous flow system will be used because of increased production and high-volume separation systems, which will increase the throughput. There are automation and process controls, but this also means higher capital costs and the use of trained personnel. It is feasible to have hybrid systems as well.
The primary byproduct is glycerin (aka glycerine, glycerol). It is a polyhydric alcohol, which is sometimes called a triol. It is a colorless and odorless liquid, which is viscous (thick-flowing) and sweet-tasting. It is non-toxic and water-soluble. Parameters to test quality are purity, color, and odor. Glycerol properties and chemical information are shown in the table below.
| Chemical name | Propane-1,2,3-triol |
|---|---|
| Chemical formula | C3H5(OH)3 |
| Molecular Weight, g/mol | 92.09 |
| Density, g/cm³ @ 20°C | 1.261 |
| Viscosity, mPa.s, @ 20°C (93% w/ water) | 1500 (400) |
| Melting point, °C (°F) | 17.9 (64.2) |
| Boiling point, °C (°F) | 290 – 297 (554-567) |
| Auto-ignition, °C (°F) | 370(700) |
| Flash Point, °C (°F) | 188 - 199 (370 - 290) |
| Food energy, kJ/g | 18 |
There are several different applications that glycerol can be used for, including the manufacture of drugs, oral care, personal care, tobacco, and polymers. Medical and pharmaceutical preparations use glycerol as a means to improve smoothness, lubrication, and moisturize – it is used in cough syrups, expectorants, laxatives, and elixirs. It can also be substituted for alcohol, as a solvent that will create a therapeutic herbal extraction.
Glycerol can be used in many personal care items; it serves as an emollient, moisturizer, solvent, and lubricant – it is used in toothpaste, mouthwashes, skin care products, shaving cream, hair care products, and soaps. Glycerol competes with sorbitol as an additive; glycerol has a better taste and a higher solubility.
Since it can be used in medical and personal care products, glycerol can also be used in foods and beverages. It can be used as a solvent, moisturizer, and sweetener. It can be used as a solvent for flavors (vanilla) and food coloring. It is a softening agent for candy and cakes. It can be used as part of the casings for meats and cheeses. It is also used in the manufacture of shortening and margarine, filler for low-fat food, and thickening agents in liqueurs.
Glycerol is also used to make a variety of polymers, particularly polyether polyols. Polymers include flexible foams and rigid foams, alkyl resins (plastics) and cellophane, surface coatings, and paints, and as a softener and plasticizer.
Unfortunately, there is already enough glycerol produced for the glycerol market. Glycerol consumption in traditional uses is 450 million lb/yr, and traditional capacity is 557 million lb/yr. If we produce glycerol from making biodiesel, it has the potential to produce 1900 million lb/yr. Therefore, we need to find a new market for glycerol, or it will be wasted in some fashion.
There is research being done to find new uses for glycerol. This includes use in additional polymers as an intermediate, conversion to propylene glycol for antifreeze, production of hydrogen via gasification, as a boiler fuel (have to remove alkali), in an anaerobic digester supplement, and for algal fermentation to produce Omega-3 polyunsaturated fatty acids.
8.4 Biodiesel Properties and Specifications
8.4 Biodiesel Properties and Specifications mjg8To ensure quality biodiesel, there are standards for testing the fuel properly to see that it meets specifications for use. ASTM (an international standards and testing group) has a method to legally define biodiesel for use in diesel engines, labeled ASTM D6751. The table below shows the test methods necessary for all the expected standards for biodiesel.
| Property | ASTM Method | Limits | Units |
|---|---|---|---|
| Ca & Mg, combined | EN 14538 | 5 max | ppm (ug/g) |
| Flash point | D 93 | 93 min | °C |
| Alcohol Control | - | - | - |
| 1. Methanol content | EN14110 | 0.2 max | % mass |
| 2. Flash point | D 93 | 130 min | °C |
| Water & Sediment | D2709 | 0.05 max | % vol |
| Kinematic Viscosity, 40°C | D445 | 1.9-6.0 | mm2/sec |
| Sulfated Ash | D874 | 0.02 max | % mass |
| Sulfur | - | - | - |
| S 15 Grade | D5453 | 0.0015 max (15) | % mass (ppm) |
| S 500 Grade | D5453 | 0.05 max (500) | % mass (ppm) |
| Copper Strip Corrosion | D130 | No. 3 max | - |
| Cetane | D613 | 47 min | - |
| Cloud Point | D2500 | report | °C |
| Carbon Residue (100% sample) | D4530 | 0.05 max | % mass |
| Acid Number | D664 | 0.50 max | mg KOH/g |
| Free Glycerin | D6584 | 0.020 max | % mass |
| Total Glycerin | D6584 | 0.240 max | % mass |
| Phosphorus Content | D4951 | 0.001 max | % mass |
| Distillation, T90 AET | D1160 | 360 max | °C |
| Sodium/Potassium, combined | EN 14538 | 5 max | ppm |
| Oxidation Stability | EN 14112 | 3 min | Hours |
| Cold Soak Filtration | Annex to D6751 | 360 max | seconds |
| For use in temperatures below -12 °C | Annex to D6751 | 200 max | seconds |
There are advantages and disadvantages to using biodiesel compared to ultra-low sulfur diesel. It has a higher lubricity, low sulfur content, and low CO and hydrocarbon emissions. This makes it good to blend with diesel from petroleum to be able to achieve the required specifications for ultra-low sulfur diesel because ultra-low sulfur diesel has poor lubricity. But as discussed previously, biodiesel has poor cold weather properties. It really depends on the location; for instance, if using biodiesel in the upper Midwest, there could be problems in the winter.
As with all materials, the production and quality of biodiesel is important. Most importantly, the transesterification reaction should reach completion for the highest production and quality. Due to the nature of the transesterification of triglycerides, a small amount of tri-, di-, and mono-glycerides remain. The figure below shows the changes in these compounds as the glycerides react to form biodiesel. Some terminology to be aware of: 1) bound glycerol is glycerol that has not been completely separated from the glyceride and is the sum of tri-, di-, and mono-glycerides and 2) total glycerol combines the bound glycerol with the free glycerol.

Glycerol content in biodiesel must be as low as possible, as ASTM standards state. The biodiesel will not technically be “biodiesel” unless ASTM standards are met, which means it is below the total glycerol specifications. High glycerol content can cause issues with high viscosity and may contribute to deposit formation and filter plugging. Crude glycerol is often a dark brown color and must be refined and purified before use elsewhere. In biodiesel preparation, brown layers will form, and, possibly, white flakes or sediments, formed from saturated mono-glycerides, will fall to the bottom of the tank the biodiesel is being stored in.
Biodiesel is also a great solvent, better than petroleum-based diesel. It can loosen carbon deposits and varnishes that were deposited by petro-diesel and can cause fuel-filter plugging when switching over to biodiesel. Filters should be changed after the first 1,000 miles with biodiesel.
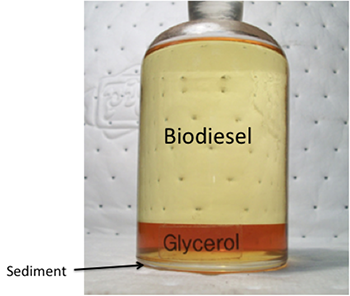
Another issue is the cold weather properties of biodiesel. These properties include cloud point, pour point, and cold soak filtration. Biodiesel can form cloud points at a much higher temperature than petro-diesel, close to the freezing point. The cloud point is the temperature at which crystals begin to form; it can cause the biodiesel to gel and flow slower than it should. Once the pour point is reached (basically completely frozen), the fuel cannot move. It depends on the normal temperature of the climate as to whether the fuel can be used or blended with petrodiesel. What can complicate it more is the saturated or unsaturated fatty acid content. High saturated fatty acid content can lead to higher fuel stability but higher pour points. High unsaturated acid content can lead to lower pour points but less stability for storing. The figure below shows a pour point comparison of biodiesels made from various oils (including fatty acid content) compared to petrodiesel. Petro-diesel pour points are significantly lower than biodiesels.

Pour point comparison of biodiesels made from various oils (including fatty acid content) and No. 1 diesel fuel.
| Biodiesel | Pour Point (Methyl Ester) ºC | Pour Point (Ethyl Ester) ºC | % Saturated Fatty Acids | % Monounsaturated Fatty Acids | % Polyunsaturated Fatty Acids |
|---|---|---|---|---|---|
| Canola | 15 | 22 | 6% | 62% | 32% |
| Safflower | 22 | 22 | 10% | 20% | 77% |
| Sunflower | 24 | 28 | 11% | 13% | 69% |
| Soybean | 25 | 30 | 15% | 24% | 61% |
| asdf | -45 | - | - | - | - |
Cetane number is also an important property for diesel fuels. Cetane number measures the point that the fuel ignites under compression, and this is what we want for a diesel engine. The higher the cetane number, the greater the ease of ignition. Most petro-diesel fuels have a cetane number of 40-50 and meet the ASTM specification for ASTM D975. In general, most biodiesels have higher cetane numbers, 46-60 (some as high as 100), and meet the specifications for ASTM D6751. Because of the higher cetane numbers of biodiesel, the engine running on biodiesel will have an easier time starting and have low idle noise. The table below shows the heat of combustions for various fuels along with their cetane number.
| Fuel | Heat of Combustion (Mj/kg) | Cetane No. |
|---|---|---|
| Methyl Ester (Soybean) | 39.8 | 46.2 |
| Ethyl Ester (Soybean) | 40.0 | 48.2 |
| Butyl Ester (Soybean) | 40.7 | 51.7 |
| Methyl Ester (Sunflower) | 39.8 | 47.0 |
| Methyl Ester (Peanut) | - | 54.0 |
| Methyl Ester (Rapeseed) | 40.1 | - |
| No. 2 Diesel | 45.3 | 47.0 |
If full-strength biodiesel is used (i.e., B100), most engine warranties will not be covered. It will also require replacing rubber seals in older engines. Blends include B2, B10, and B20 (2%, 10%, and 20% biodiesel, respectively). Adding biodiesel as a blend with ultra-low sulfur should improve lubricity for ultra-low sulfur diesel fuel, which will improve engine wear. Emissions of hydrocarbons, CO, NOx, and particulate matter are similar to petrodiesel fuels, although can be reduced in some cases.
Biodiesel is stored very similarly to petrodiesel. It is stored in clean, dark, and dry environments. It can be stored in aluminum, steel, fluorinated polyethylene, fluorinated polypropylene, and Teflon types of containers. It is best to avoid copper, brass, lead, tin, and zinc containers.
In another lesson, we will discuss the economics behind using biodiesel.
8.5 Summary and Final Tasks
8.5 Summary and Final Tasks mjg8Summary
In this lesson, you’ve learned about how to make biodiesel from vegetable oils. You’ve had the opportunity to see how it is made and that it’s fairly simple to make. You’ve been provided with information on properties and how biodiesel is typically used. In a future lesson, you will be provided with information on the economics behind biodiesel and ethanol, as well as determining how much energy it takes to make biodiesel, versus the amount of energy that is produced. The additional information is important to determining the use of, and best practices for making, alternative fuels.
Lesson Objectives
By the end of this lesson, you should be able to:
- explain the chemistry of vegetable oil biodiesel, and several ways to make it;
- explain how to utilize biodiesel in a diesel engine;
- evaluate the best uses of biodiesel.
References
Scott W. Pryor 1*, B. Brian H 2, J.H. Van Gerpen 2 1. Department of Agricultural and Biosystems Engineering, North Dakota State University, 2. Department of Biological and Agricultural Engineering, University of Idaho, BEEMS Module B4, Biodiesel, USDA Higher Education Challenger Program, 2009-38411-19761. Contact: Scott Pryor, Scott.Pryor@ndsu.edu
Reminder - Complete all of the Lesson tasks!
You have reached the end of this Lesson! Double-check the Road Map on the Lesson Overview page to make sure you have completed all of the activities listed there before you begin the next Lesson.
Questions?
If there is anything in the lesson materials that you would like to comment on, or don't quite understand, please post your thoughts and/or questions to our Throughout the Course Questions & Comments discussion forum and/or set up an appointment during office hours. While you are there, feel free to post responses to your classmates if you can help.
